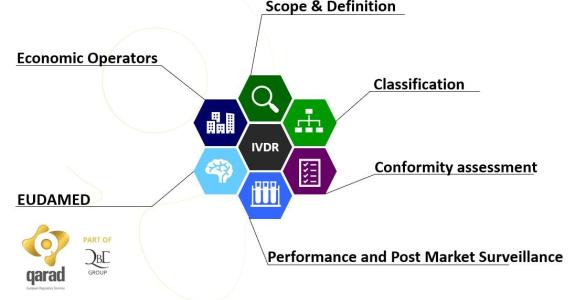
26 May 2022 is the official date of application for the Regulation 2017/746 on in vitro diagnostic medical devices (IVDR), thereby repealing the old IVD directive 98/79/EC. There are still many obstacles to overcome and unclarities to address for IVD manufacturers and suppliers to the IVD industry. In need for clarity? Join us on 24 November!
What is meant exactly with 'in-vitro diagnostic medical device'? The term covers any medical device which is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, piece of equipment, software or system, whether used alone or in combination, intended by the manufacturer to be used in vitro for the examination of specimens, including blood and tissue donations, derived from the human body, solely or principally for the purpose of providing information on, make predictions of or monitor the condition and responses of the human body.
This is what the IVDR regulation is about. This regulation lays down rules concerning the placing on the market, making available on the market or putting into service of in-vitro diagnostic medical devices for human use and accessories for such devices in the European Union. This regulation also applies to performance studies concerning such in-vitro diagnostic medical devices and accessories conducted in the Union.
Information session in cooperation with Qarad
Within the context of the next supervisory group meeting of the COOCK project 'Medical diagnostics goes micro and smart' on 24 November, a seminar will be organised by one of the member companies. Qarad - part of the QbD group - is specialized in Regulatory Affairs and Quality Systems for the IVD industry and will tell us more of the new IVDR regulation. During this seminar we will focus on the application of the Regulation on microfluidic chips and on their conditioning and read-out devices meant for diagnostic testing. An expert in the IVD field from Qarad will provide insights into these regulations and you will have the opportunity to ask questions related to your specific project or situation.
Would you like to participate in our session at our site in Leuven on 24 November (11:00-13:00)? Check our agenda and register!